Copyright 2012 neutronsources.org | All rights reserved. | Powered by FRM II | Imprint / Privacy Policy
Neutrons reveal potential dangers of gold nanoparticles – pharma’s drug delivery agent of the future
07.06.2013
• High concentrations of positively charged gold flakes shown to penetrate deep into the cell’s outer membrane destroying it, whilst negatively charged particles can stabilise the membrane
• First finding from research project at ILL to help better understand safe handling and use of potentially valuable nanoparticles
Scientists working at the Institut Laue-Langevin have shown that the charge of gold nanoparticles, identified by major pharmaceutical companies as a drug delivery agent of the future for the treatment of cancer, affects how they interact with our cell’s protective outer wall. These crucial insights, published in Langmuir, provide a first step in the effective design of safe nanoparticles for biomedical applications and the practices and procedures for their secure handling in a variety of other consumer products.
The growing use of nanoparticles, tiny flakes of material, 1 millionth the size of a grain of sand, in a wide range of commercial products, such as clothing, food storage containers, pharmaceuticals, cosmetics, tyres, electronics and optical devices, is controversial. Common nanoparticles, such as gold, silver and copper, easily penetrate organic membranes, (cell walls, …) creating potentially significant impacts on human health and the environment. However, there is one area where their toxicity might prove useful and even life-saving.
A major challenge in modern medicine is finding delivery agents capable of targeting and penetrating cells to transport drugs directly inside the infected tissue. The search for the right vehicle has led to a new field of research, ‘nanomedicine’, where nanoparticles could be programmed to target cancerous cells for example, reducing or even eliminating the need for surgery.
Of all the nanoparticles available to medical science, one in particular has become a focus of research amongst major pharmaceutical companies – gold. AstraZeneca last year announced a new research project to look at a new nanomedicine, CYT-6091, based on gold nanoparticles [1].
Gold nanoparticles make particularly good delivery vehicles because:
• They are easy to ‘load-up’ with other molecules, such as existing cancer drugs
• They are easy to produce
• They are chemically stable inside the body
• They offers a unique set of optical, electronic and thermal properties, which means they can be ‘switched on’ inside the body very easily when they arrive at the right location.
However, at present we don’t understand in any detail the interaction mechanisms between nanoparticles and our cell’s outer defences – the cell membrane. Without this it is impossible to determine how dangerous they are and whether their ability to penetrate and destroy cells can ever be harnessed for good ends, such as in the fight against cancer.
One thing that is known is that there is a complex set of parameters that influence this interaction, including the nanoparticle’s shape, size, composition and charge. But a systematic study that shows how the interaction depends on these parameters at a molecular level has up until now been lacking.
To start to address this, a research team from the Institut Laue-Langevin (ILL), the University of Illinois and the Australian Nuclear Science and Technology Organization used the ILL’s neutrons and world-leading neutron scattering instruments to investigate, on a molecular level, the physical changes undergone by our cell walls as they come into contact with gold nanoparticles of different charge.
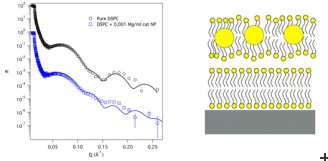
2 nm diameter gold nanoparticles had either cationic (positively charged) or anionic (negatively charged) groups added to their surface. To mimic the cell membrane, the research team used two double layers of fatty lipid molecules held 20-30 Å on top of each other that together produced the dynamic properties seen in cell membranes. The scientists then applied neutron reflectometry techniques at the ILL to accurately model the nanoparticle – cell membrane interaction on a fraction of a nanometre scale.
What they found was that the nanoparticle surface charge does indeed play a significant role in determining their interaction with our cells membranes. Cationic nanoparticles pass straight through the lipid membrane and embed themselves deeply within the floating bilayer, destabilising the entire membrane structure sufficiently to completely destroy the cell at higher concentrations. In contrast, anionic nanoparticles do not penetrate the lipid membrane at all. Rather, at given concentrations they hinder membrane decomposition helping it withstand the sort of extreme conditions, such as elevated pH, that would normally significantly destabilise it.
Quotes
Dr. Sabina Tatur: “Our research shows the potential risks of the premature use of nanoparticles in everyday goods before all aspects of their safety have been defined and considered. With a steadily increasing addition of nanomaterials in everyday consumer products, we hope to raise awareness about the growing importance of systematic research in ‘nanosafety’. Nanomaterials are indeed promising candidates for countless exciting applications – but only if they are shown to be harmless for the health and environment.”
Dr. Giovanna Fragneto: “This work represents an exciting application of the model membranes, the development of which has been pioneered during the last 15 years at the ILL. Sample preparation and the biological relevance of the models you create are critical factors for the study of interactions at the membrane level with neutron techniques and at the ILL we are able to provide our beam scientists with world-leading sample preparation systems.”
Dr. Rob Barker: “The fact these nanoparticles can attack cells’ outer protective layers so effectively is both concerning from a general health point of view but also potentially exciting in terms of future medical treatment. What is clear is understanding how these interactions take place is key, and this discovery around the significant role that charge plays in this process will give impetus to developing methods and procedures for the safe handling and use of these valuable materials going forward.”
Dr. Marco Maccarini: “Neutrons are an ideal tool for studying biological materials, particularly their reactions and interactions on surfaces and across interfaces. They are highly sensitive to lighter atoms, such as carbon, hydrogen that make up organic molecules, and can distinguish between them very easily. Also through the use of isotopic substitution, we at the ILL can vary the contrast between different regions of the system, highlighting the position of each component by the different degrees of scattering. As one of the world’s brightest neutron sources, the ILL has a long history of modelling important micro and nano-scale processes of biological and biophysical interest and providing ground-breaking insights that inform the next generation of treatments.”
Re.: Langmuir, DOI: 10.1021/la401074y
Contact: Mr James Romero +44 8456801866
Notes to editors
1. Astrazeneca announce research into new cancer nanomedicine based on gold nanoparticles
2. The Institut Laue-Langevin (ILL) is an international research centre based in Grenoble, France. It has led the world in neutron-scattering science and technology for almost 40 years, since experiments began in 1972. ILL operates one of the most intense neutron sources in the world, feeding beams of neutrons to a suite of 40 high-performance instruments that are constantly upgraded. Each year 1,200 researchers from over 40 countries visit ILL to conduct research into condensed matter physics, (green) chemistry, biology, nuclear physics, and materials science. The UK, along with France and Germany is an associate and major funder of the ILL.